When a patient picks up a generic medication, they expect it to work just like the brand-name version. But what if it doesn’t? What if they develop a rash, feel dizzy after years of stable use, or have trouble breathing-something that never happened before? These aren’t just coincidences. They’re adverse events, and pharmacists are often the first-and sometimes only-healthcare professional who notices them.
Why Generic Medications Need Extra Attention
Generic drugs are supposed to be bioequivalent to their brand-name counterparts. That means they contain the same active ingredient, in the same strength, and are meant to have the same effect. But bioequivalence doesn’t mean identical. The inactive ingredients-fillers, dyes, preservatives-can vary. And those differences? They can trigger reactions in sensitive patients.A 2022 study in the Journal of the American Pharmacists Association found that 1 in 5 patients who switched to a generic version reported a new or worsening side effect. Many dismissed it as "just adjusting," until the pharmacist asked the right questions. One patient in Portland developed severe headaches after switching to a generic version of a blood pressure med. The brand-name version had used a lactose-based filler. The generic used cornstarch. The patient had a rare, undiagnosed corn sensitivity. No prescriber caught it. The pharmacist did-and reported it.
That’s why pharmacists can’t assume generics are automatically safe. The assumption that "it’s the same" leads to under-reporting. Health Canada estimates only 5-10% of all adverse drug reactions get reported. For generics, that number may be even lower because providers don’t suspect anything’s wrong.
What Pharmacists Are Legally Required to Do
There’s no federal law in the U.S. that forces pharmacists to report adverse events. But that doesn’t mean they’re off the hook. In many states, it’s part of professional responsibility.In British Columbia, pharmacists are legally required to report any suspected adverse drug reaction to Health Canada. They must also notify the prescribing provider and update the patient’s PharmaNet record. New Jersey requires consultant pharmacists to document and report adverse events before the end of their shift. These aren’t suggestions-they’re rules.
The FDA doesn’t require reporting, but it strongly urges it. The agency’s FAERS database (FDA Adverse Event Reporting System) gets over 2 million new reports each year. Most come from pharmaceutical companies, but those companies only get the reports because pharmacists, nurses, and doctors passed them along.
Pharmacists are the bridge between the patient and the system. If you don’t report, the data doesn’t exist. And without data, regulators can’t spot patterns-like a new generic causing liver inflammation in elderly patients, or a specific dye triggering anaphylaxis in kids.
What Counts as an Adverse Event
Not every side effect is reportable. The FDA defines a serious adverse event as one that:- Results in death
- Is life-threatening
- Requires hospitalization
- Causes permanent disability
- Leads to congenital malformation
- Needs medical intervention to prevent serious harm
But non-serious events matter too. If a patient says, "This new generic makes me nauseous every morning," and it didn’t happen with the brand, that’s worth documenting. Why? Because if 10 other patients report the same thing, it becomes a signal. A pattern. A warning.
The Ontario College of Pharmacists defines an adverse drug reaction as "a harmful and unintended effect"-no matter how minor. That’s the standard to aim for. Don’t wait for hospitalization. If it’s unexpected, unusual, or worse than what’s listed in the package insert, report it.
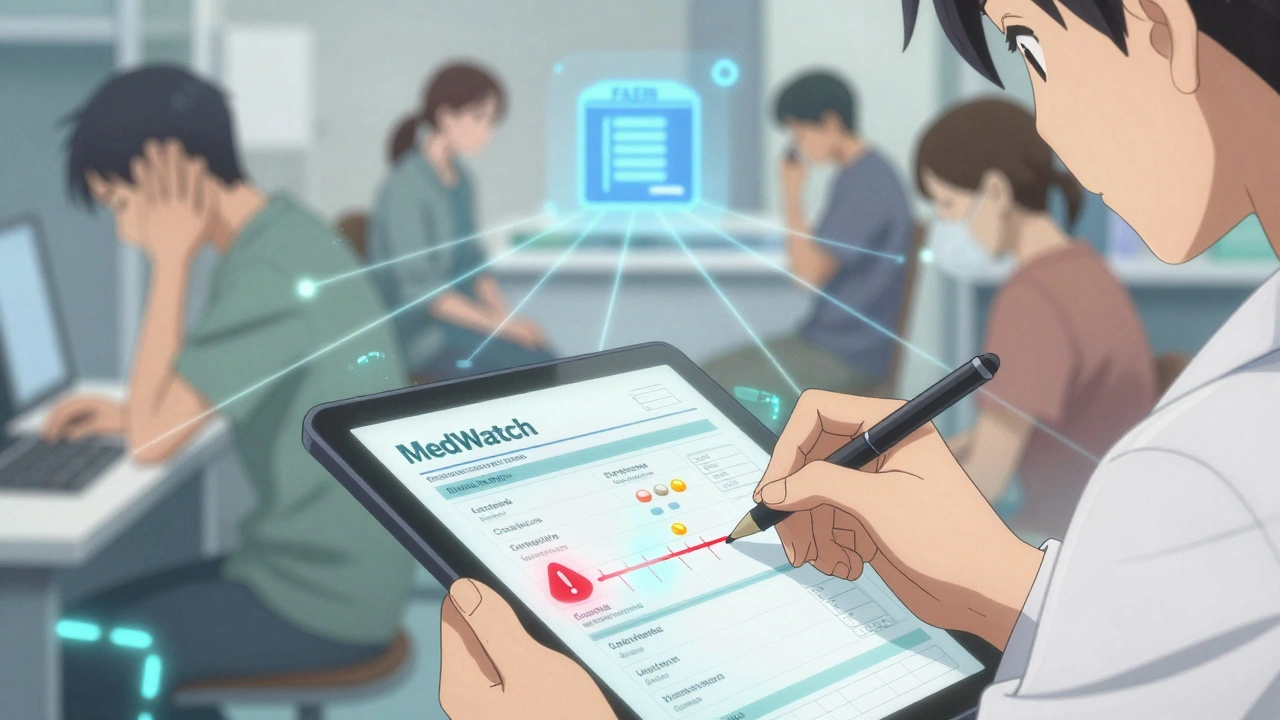
How to Report: The Real-World Process
Reporting isn’t complicated, but it’s often messy. Here’s how it actually works:First, document everything. Write down the patient’s name (or initials if privacy is a concern), the drug name (brand and generic), dose, start date, when symptoms began, what they are, and how long they lasted. Note any other medications or supplements the patient is taking. This isn’t just for the report-it’s for your own records.
Next, decide if it’s reportable. If it’s serious, report it immediately. Use the FDA’s MedWatch Online portal. It takes about 15 minutes. You don’t need to prove causation-just suspicion. The FDA’s job is to investigate, not for you to diagnose.
For non-serious but unusual events, report to the manufacturer. Most generics are made by large companies like Teva, Mylan, or Sandoz. They have hotlines or online forms. Send them your report. They’re legally required to forward it to the FDA.
Community pharmacists often use paper forms or email. Hospital pharmacists usually report through EHR systems with built-in reporting tools. Either way, make sure it’s documented in the patient’s file. If another pharmacist sees the same patient later, they need to know.
A 2021 survey by the National Community Pharmacists Association found that 78% of pharmacists spend 15-30 minutes per report. That’s a lot of time. But the cost of not reporting? Higher. Unreported events mean delayed recalls, missed warnings, and preventable harm.
Why Reporting Is So Hard-and How to Overcome It
Time is the biggest barrier. Pharmacies are busy. Patients are waiting. You’re juggling refills, insurance issues, and counseling. Adding another task feels impossible.But here’s the truth: you’re already doing the work. You’re listening. You’re noticing. You’re asking, "Did this start after the switch?" That’s half the battle. The reporting part? It’s just the paperwork.
Here’s how to make it easier:
- Keep a printed checklist by the counter: "Did the patient switch generics? Did symptoms change? Is this unexpected?"
- Use templates. Save a pre-filled MedWatch form in your computer. Just update the details.
- Team up. If you’re part of a group practice, assign one person to handle reports each week.
- Ask patients to sign a consent form for reporting. Many don’t realize their feedback helps others.
And don’t wait for training. The British Columbia Pharmacists Association says lack of awareness is a top reason for under-reporting. You don’t need a seminar. Just read the FDA’s MedWatch guidelines. It’s one page.
The Bigger Picture: Why This Matters
Every report you file is a piece of a puzzle. The FDA’s FAERS database has over 24 million entries since 1968. That’s not just numbers-it’s lives saved.Remember the case of the generic metformin? In 2020, reports started coming in about patients developing a strange metallic taste and nausea. No one thought much of it-until 120 reports clustered around one manufacturer. Turns out, a new manufacturing process created a contaminant. The FDA issued a recall. That wouldn’t have happened without pharmacists speaking up.
Generic drugs save the U.S. healthcare system over $300 billion a year. But savings shouldn’t come at the cost of safety. Pharmacists are the last line of defense. We’re the ones who hand the bottle to the patient. We’re the ones who see them again when things go wrong.
By 2025, experts predict 75% of U.S. states will make adverse event reporting mandatory for pharmacists, following British Columbia’s lead. The European Medicines Agency saw a 220% increase in reports after making it mandatory in 2012. The data speaks for itself.
This isn’t about bureaucracy. It’s about trust. Patients trust you to know what’s safe. When you report, you’re not just filling out a form-you’re protecting the next person who walks through the door.
What Happens After You Report
After you submit a report, it goes into FAERS. The FDA analyzes trends. If a pattern emerges, they may:- Issue a safety alert
- Require label changes
- Ask the manufacturer to investigate
- Initiate a recall
You won’t always hear back. That’s normal. But your report matters. Even if it’s the only one. One report can be the spark.
Some pharmacists worry about liability. But reporting is protected under federal law. You’re acting in good faith. You’re doing your job.
And if you’re ever unsure? Call the FDA’s MedWatch hotline. They’ll walk you through it. No judgment. No hassle. Just help.
Do pharmacists have to report adverse events by law?
There’s no federal law in the U.S. requiring pharmacists to report adverse events, but several states like British Columbia and New Jersey have made it mandatory. Even where it’s not required, professional ethics and FDA guidelines strongly encourage reporting, especially for serious or unexpected reactions. Failing to report known serious events can be considered negligence.
Can I report an adverse event even if I’m not sure it’s caused by the medication?
Yes. The FDA doesn’t require proof of causation. If you suspect a medication might be involved-even if another cause is possible-you should report it. The goal is to catch patterns early. Many drug safety alerts started with just one or two reports from pharmacists who said, "This doesn’t seem right."
What’s the difference between a side effect and an adverse event?
A side effect is a known, expected reaction listed in the drug’s labeling-like drowsiness from antihistamines. An adverse event is any harmful, unintended reaction that wasn’t expected, is more severe than listed, or occurs after switching to a generic version. Even if it’s mild, if it’s new or unusual, it’s worth reporting.
How do I report an adverse event from a generic drug?
Use the FDA’s MedWatch Online portal for serious events. For non-serious but unusual reactions, report directly to the manufacturer (they’re required to forward it to the FDA). Always document the patient’s details, the drug name (brand and generic), when symptoms started, and what they are. Keep a copy in the patient’s record.
Why are generic medications more likely to cause under-reported adverse events?
Because providers and patients assume generics are identical to brand-name drugs. When a patient has a reaction, they often blame the condition, not the medication. Pharmacists are often the only ones who know the patient switched generics. Without that context, the link gets missed. That’s why pharmacist reporting is critical-it fills the gap in awareness.



12 Comments
Bonnie Youn December 1, 2025 AT 15:53
Finally someone says it loud and clear - pharmacists are the unsung heroes catching these hidden reactions
My aunt got a rash from a generic statin and the doctor blew it off until the pharmacist pushed
Now she’s back on brand and fine
Report everything - even if it seems small
Karandeep Singh December 3, 2025 AT 03:12
Generic same active ingredint. If u get reaction its ur body not the drug. Stop overreacting
Alexander Williams December 4, 2025 AT 06:44
The bioequivalence standard is a regulatory fiction. The FDA allows up to 20% variation in AUC and Cmax - that’s not equivalence, it’s tolerance. And when you layer in excipient variability across 12 manufacturers of the same generic, you’re not dealing with a pharmaceutical product - you’re dealing with a lottery ticket. The notion that pharmacists are the ‘last line of defense’ is a comforting myth. The system is designed to absorb harm until the volume triggers a signal - which takes years. We’re not protecting patients. We’re just documenting the slow bleed.
Scotia Corley December 4, 2025 AT 09:52
While the article appropriately emphasizes the professional responsibility of pharmacists, it fails to address the systemic erosion of clinical autonomy under corporate pharmacy models. In many chains, pharmacists are incentivized to minimize counseling time and maximize script throughput. Reporting adverse events requires documentation, follow-up, and time - none of which are reimbursed. Until regulatory bodies recognize this as a labor issue and not merely an ethical one, reporting rates will remain abysmal. The burden cannot be placed on the frontline worker when the infrastructure is broken.
Suzanne Mollaneda Padin December 5, 2025 AT 08:15
I’ve been a pharmacist for 18 years and I can tell you - the biggest barrier isn’t time, it’s fear. Fear that the patient will think you’re overstepping. Fear that the prescriber will get defensive. Fear that you’ll be wrong. But here’s what I’ve learned: patients appreciate it when you care enough to ask. I keep a simple checklist on my counter: Switched? New symptom? Worse than before? If yes - document. If unsure - report. You don’t need to be certain. You just need to be curious. And that curiosity? It saves lives.
Edward Hyde December 5, 2025 AT 19:25
Oh great. Another ‘pharmacist as hero’ piece while Big Pharma laughs all the way to the bank.
Let’s be real - generics are just brand-name drugs with cheaper fillers and a bigger profit margin for the manufacturer.
And now we’re supposed to be the ones doing the dirty work of catching their toxic byproducts?
Meanwhile, the FDA approves 90% of generic applications without even looking at the actual manufacturing site.
Report all you want. No one’s gonna fix the system. Just keep handing out pills and pretending you’re making a difference.
Margaret Stearns December 6, 2025 AT 21:34
i read this and thought about my mom. she switched to a generic thyroid med and started having panic attacks. we thought it was stress. the pharmacist asked if she switched meds and she said yes. he said try the brand again. it worked. i didnt know you could report stuff like that. thanks for explaining how. i’m gonna print this out and give it to my pharmacy.
Mary Ngo December 8, 2025 AT 17:44
Have you ever wondered why the FDA doesn’t require manufacturers to use the same excipients across all generics? Or why the same dye used in one generic causes anaphylaxis in 3 states but is fine everywhere else? This isn’t about safety - it’s about control. The pharmaceutical-industrial complex uses excipient variability as a tool to fragment adverse event reporting. If every generic had identical fillers, patterns would emerge too quickly. They need the chaos. They need you to think it’s random. It’s not. It’s engineered. Report your data - but don’t expect it to change anything. The system is designed to bury you in paperwork while the real culprits remain invisible.
Kenny Leow December 9, 2025 AT 05:38
As someone who’s worked in both community and hospital pharmacy, I’ve seen this play out a hundred times. The key is consistency. I use a pre-filled MedWatch template saved as a PDF. I just copy-paste the patient’s initials, drug name, and symptom. Takes 3 minutes. I’ve filed 47 reports in 3 years. Two led to manufacturer investigations. One led to a label update. You don’t need to be a hero. Just be consistent. And yes - I still get yelled at for asking if they switched generics. But I’d rather get yelled at than miss something that kills someone. 🙏
Kelly Essenpreis December 10, 2025 AT 21:55
Why are we even talking about this like it’s new? This has been going on since the 80s. The FDA approved the first generic in 1984 and since then we’ve had dozens of recalls tied to excipients. But no one wants to admit that the ‘same drug’ myth is a lie. We’re just pretending generics are safe because it saves money. And now we’re putting the burden on pharmacists to fix a problem created by politicians and CEOs. Wake up. This isn’t about reporting. It’s about systemic corruption and we’re all just pawns.
Lauryn Smith December 11, 2025 AT 14:40
My favorite thing about this post is how it doesn’t make you feel guilty - it makes you feel capable. You don’t have to be perfect. You don’t have to know everything. Just ask the question: ‘Did this start after the switch?’ That’s it. That’s the whole key. I’ve had patients cry because they thought they were crazy for feeling different. And then we report it. And sometimes - just sometimes - someone else gets saved because of it. You’re not just a pharmacist. You’re a witness. And that matters.
James Allen December 12, 2025 AT 03:36
Let’s be honest - if you’re a pharmacist in a chain store and you spend 15 minutes on a report, your manager will give you a side-eye and ask why you didn’t help the 12 people waiting in line.
Meanwhile, the corporate HQ is making $400 million off that same generic.
So yeah - report if you can.
But don’t pretend this is about ethics.
This is about capitalism eating the healthcare system alive.
And we’re just the ones left holding the clipboard.